Anodizing is an electrochemical process that converts the metal surface into a decorative, durable, corrosion-resistant, anodic oxide finish. Aluminium is ideally suited to anodizing, although other nonferrous metals, such as magnesium and titanium, also can be anodized.
Anodizing, used mostly on aluminium and zinc, is an electrochemical process that deposits an oxide coating on the surface of the metal, which provides a certain amount of corrosion resistance and can be dyed in brilliant colours. Plain anodizing is widely used for aluminium extrusions and decorative parts. Hard anodizing is used for cookware.
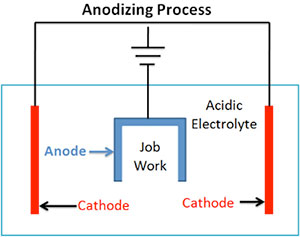
(Image source)
Generally speaking anodizing costs less than powder coating or painting. Due to the translucent property of anodizing, it gives aluminium a deeper, richer metallic appearance than many other organic coatings. Anodising is much better for aluminium, especially in high-traffic areas where a coating is prone to abrasive cleaners and other forms of physical abuse. Anodised aluminium metals cannot chip, peel, blister, or flake. This is because the coating is part of the metal itself.
Hard anodized aluminium is used in a variety of cookware. The process keeps the metal from reacting with acidic foods and provides a hard, smooth surface that is very durable. Aluminium conducts heat well and is a less expensive metal.


 Exmples of anodised parts.
Exmples of anodised parts. Aluminium Chassis.
Aluminium Chassis. Hard anodized cookrware.
Hard anodized cookrware.